Plasoma consists of
a cold plasma pad and a cold plasma pulser
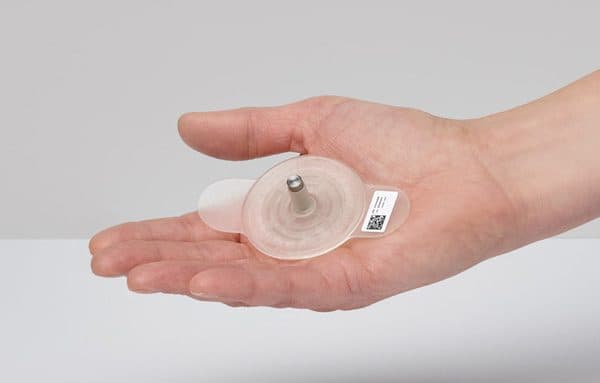
PLASOMA cold plasma pad is flexible and is fixed over the wound. After one push on the button, the cold plasma pulser will ignite the cold plasma below the pad. The current pad has an active cold plasma area with a diameter of 3 cm. This size fits most of the diabetic foot ulcers and open leg wounds. More cold plasma pad sizes will be developed over time.
PLASOMA cold plasma pulser sends an electric current to the pad to ignite the cold plasma. The progress of the treatment can be followed on the display. The cold plasma pulser is intelligent: it monitors continuously the treatment process and automatically stops the treatment in the event of irregularities.

PLASOMA cold plasma treatment
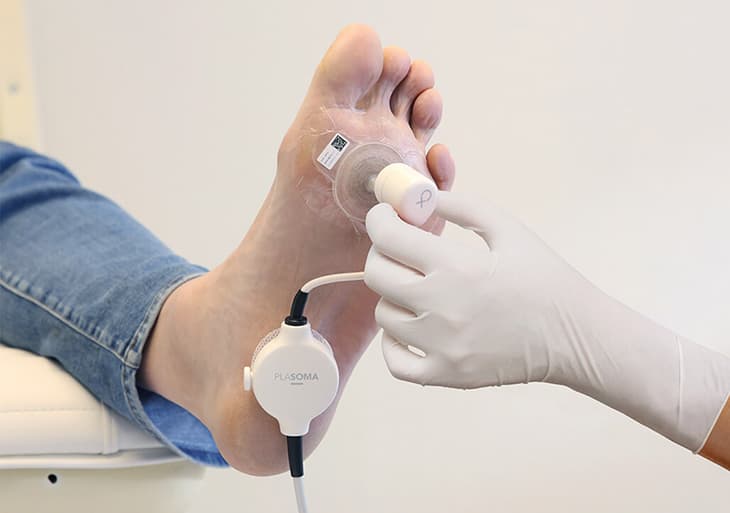
Underneath the cold plasma pad cold plasma is created in the wound. The pad is connected to the pulser that sends energy to the pad to create cold plasma. The treatment will start and lasts 2 minutes. Once finished, the pad is removed and disposed. The procedure will be repeated regularly until the wound has healed. The wound receives standard wound care next to PLASOMA cold plasma treatment.
PLASOMA features & benefits
- Effective: strong plasma with electric field + current directly in the wound
- Safe: closed system so plasma cannot escape + counter electrode ensuring defined return path
- User-friendly: 2-minutes, handsfree. Easy to fit into existing treatment regimens
- Portable: suited for use in clinic and at home
- Versatile: multiple indications incl. diabetic foot ulcers, leg ulcers, pressure ulcers, burn wounds, post-surgical ulcers, etc.. Flexible treatment regimens (e.g. 1x / 2x per week)
![]()

